Are you accidentally affecting your own nutrition program with how you use Boron and Molybdenum? And the AgriWiz experiment.
Why are some farmers reporting issues with unexpected precipitation of solid matter at the bottom of tanks, causing filters and emitters to block? Why do some leaf analyses consistently show unchanged, low levels of Zinc or other micronutrients despite farmers regularly increasing the amount being included in the fertigation mix? Could the culprits possibly be Molybdenum (Mb) and Boron (B) in the Tank Mix?
The AgriWiz Experiment:
An extensive literature search did not produce enough information, so AgriWiz decided to carry out our own experiments with commonly used fertilizer ingredients. It must be stressed that we used maximum solution concentrations to try and create “worst case” scenarios and increase the chance of precipitation.
What we did:
- We made saturated solutions of chemicals containing cations (usually metals) and anions that are present or required in commonly used fertilizers.
- We then calculated the volume of each cation and anion solution that was most likely to interact when mixed.
- We mixed the two together and recorded (and photographed) the result – immediately and after 24 hours, 48 hours, 1 week and 2 weeks.

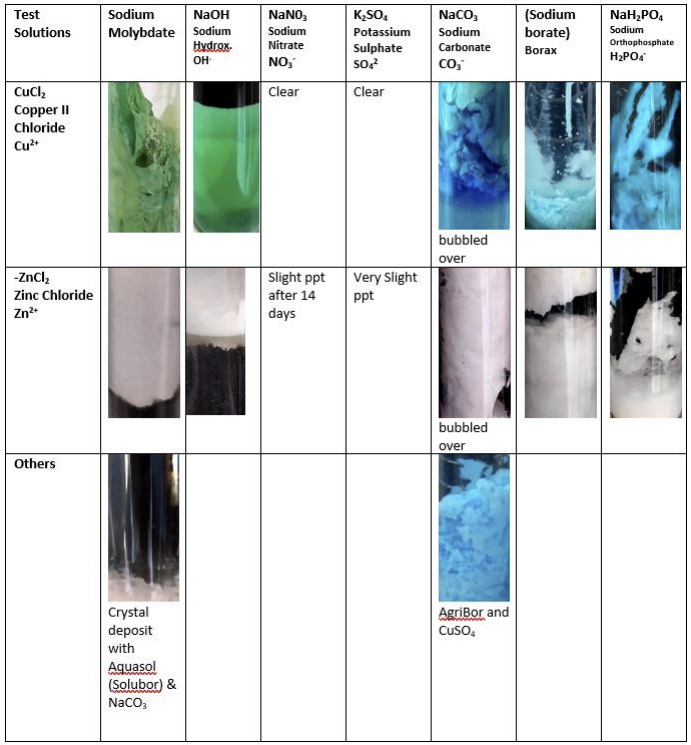
The Results and Conclusions:
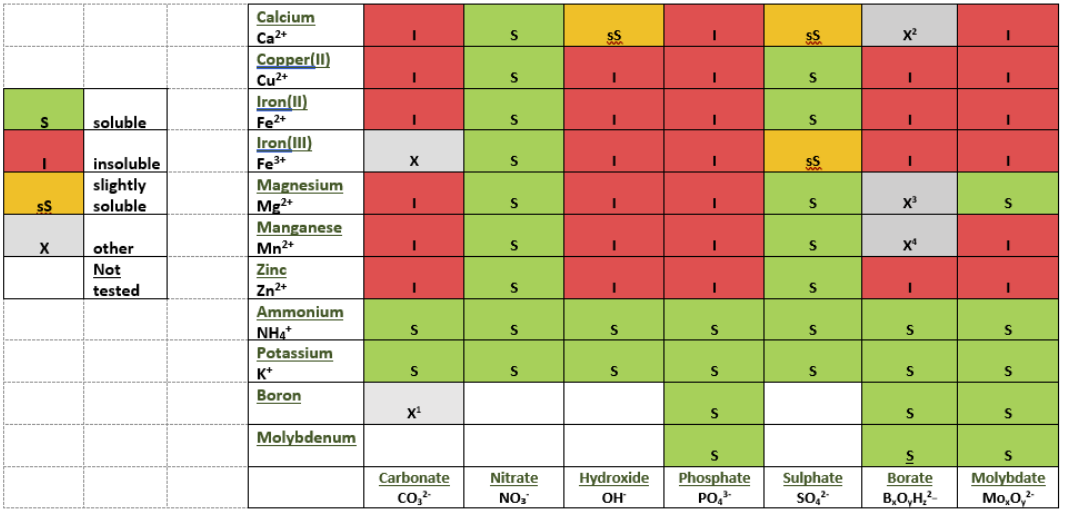
An immediate reaction between any particular cation and anion was taken to indicate a risk of precipitation in the creating of Tank Mixes, especially with the impending use of tank concentrates. Precipitation may not occur in the Tank Mix Station, but extreme care should be taken. The results are shown in this colored table and in the photographs above.
A Basic Reminder about Solutions, Cations, and Anions:
A chemical compound (or salt) such as Copper Sulphate (CuSO4) is made up of 2 components or IONS, which are held together in the solid state by their electrical charge. The CATION is the positive particle (Cu2+) and the ANION is the negative particle (SO42-). In solution, these particles disassociate and are free to be taken up by the plant roots or to interact with other ions present in the solution. Some cations and anions are more strongly attracted to each other than others and, if the resulting compound is insoluble, precipitation can occur. Although many fertilizer products are described as “soluble in water”, very few of these are applied as solutions of a single fertilizer – in common agricultural practice, several required nutrients are mixed in solution, in storage tanks, and calculated volumes are injected into the irrigation system for application to the crop.
Solubility is affected by:
- Concentration (amount being dissolved)
- The quality of the irrigation water e.g. pH or high levels of calcium or hydroxides may have an adverse effect
- The presence of other chemicals in the solution
- pH (previously dissolved chemicals may change the pH)
- Temperature of the water. Most chemicals dissolve more easily in hot water but certain compounds, such as Calcium Nitrate, cause a dramatic decrease in temperature when dissolved (endothermic reaction)
- Inadequate mixing and ongoing agitation.
Potential Problems and How to Reduce Them:
There are certain fundamental rules that it is recommended be followed when deciding on a fertigation tank mix:
- The onus is on the farmer to perform a “jar test” (details below) to check for precipitation issues. This is increasingly important as higher concentrations of fertilizer are mixed.
- A minimum of 3 tanks is needed in a multi-tank system to reduce the chance of problems – i.e. Sulphates, Phosphates and Calcium.
- Do not mix phosphates (PO43-), Molybdenum (Mb) or Boron with anything else except each other (or have a separate tank for each).
- Fertilizers containing Calcium, Copper, Iron, Magnesium, Manganese, or Zinc (Ca2+, Cu2+, Fe3+, Mg2+, Mn2+, Zn2+) should not be mixed with fertilizers containing Phosphates, Carbonates, Hydroxides (PO43-, CO32-, OH-) or Molybdates.
- Calcium (Ca2+) should never be mixed with fertilizers containing Sulphate (SO42-). Calcium can be mixed with Ammonium Nitrate but NOT if there is Sulphate present.
- Boron should not be mixed with Iron, Copper, or Zinc. (Magnesium and Manganese with caution.)
- Iron (Fe) should generally be used as a chelate.
N.B. Chemical deposits blocking drippers and pipes will not be cleared by flushing with hydrogen peroxide – that is only effective on organic deposits such as algae.
The Jar Test:
- Calculate the required quantity of each fertilizer in g per litre. = Recommended amount in kg per tank Volume of tank in litres x 1000
- Measure 1 l of irrigation water into a suitable clear container e.g. glass jar with lid (big enough to agitate contents).
- Add weighed fertilizers, one at a time, mixing well after each addition.
- Let solution stand for 15 minutes after mixing, then mix/shake again and observe results:
- If the container is warm or cold to touch, the mixture is incompatible.
- If layers form or there is cloudiness or sludge, the mixture is incompatible.
- Check again over a period of a few days.
Chelation:
AgriWiz recommends that Iron always be used in chelated form as it is highly reactive. Chelated products are more expensive, and their effectiveness is limited by the pH of the solution and the presence of other ions. These chelate types are most stable in the pH ranges shown:
- EDTA 3-6
- DTPA 3-6.5
- EDDHA 3-10
EDTA has an order of chelation and an ion lower down this list may be displaced by one above it, especially at high concentrations:
- Iron (Ferric)
- Copper
- Cobalt
- Iron (Ferrous)
- Zinc
- Manganese
- Magnesium
- Calcium”
